High-energy water release model applied to adsorption of guest molecules
The non-covalent complexation of small organic molecules in aqueous media remains challenging because water as a solvent is highly competitive for any polar non-covalent bond. Thus, artificial receptor candidates designed to strength en direct receptor-ligand interactions (Emil Fischer’s lock and key model) typically afforded only moderate binding affinities in water, by no means reaching that of protein receptors. Unlike the lock-and-key model, the high-energy-water release model states that poorly solvated host cavity are a guar antor for strong host-guest binding, where as direct host-guest interactions are often of secondary importance.1 We believe, it can be extended to systems that bind small molecules in concave cavities in water, e.g. to protein-ligand binding and to functional mate rials, designed for uptake of molecules in aque ous media,such as metal-organic frameworks (MOFs), covalent organic frameworks (COFs) and imprinted polymers.
At the end of the YIN grant project, we found first evidence that the high-energy water release model is also applicable to nanoporous MOFs.
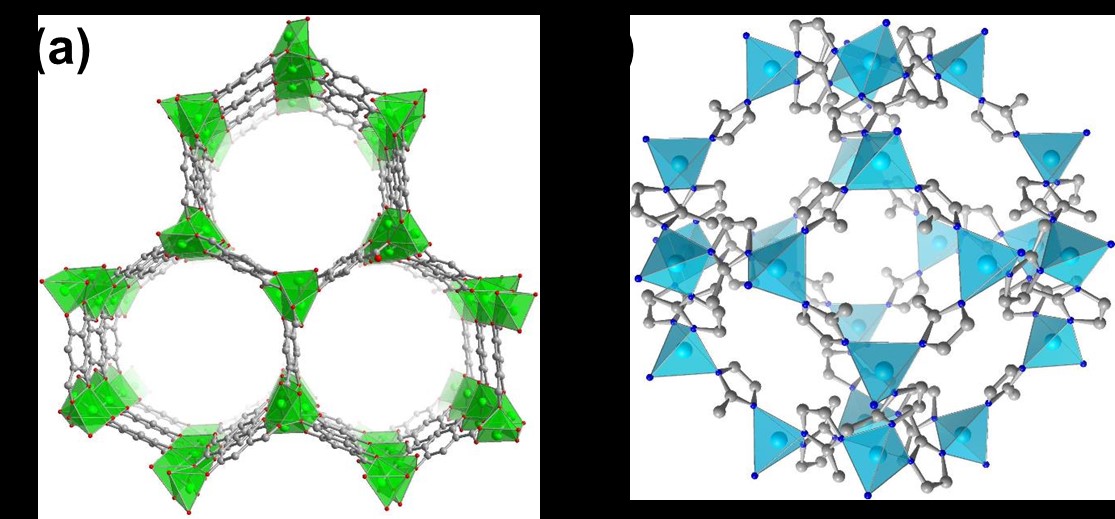
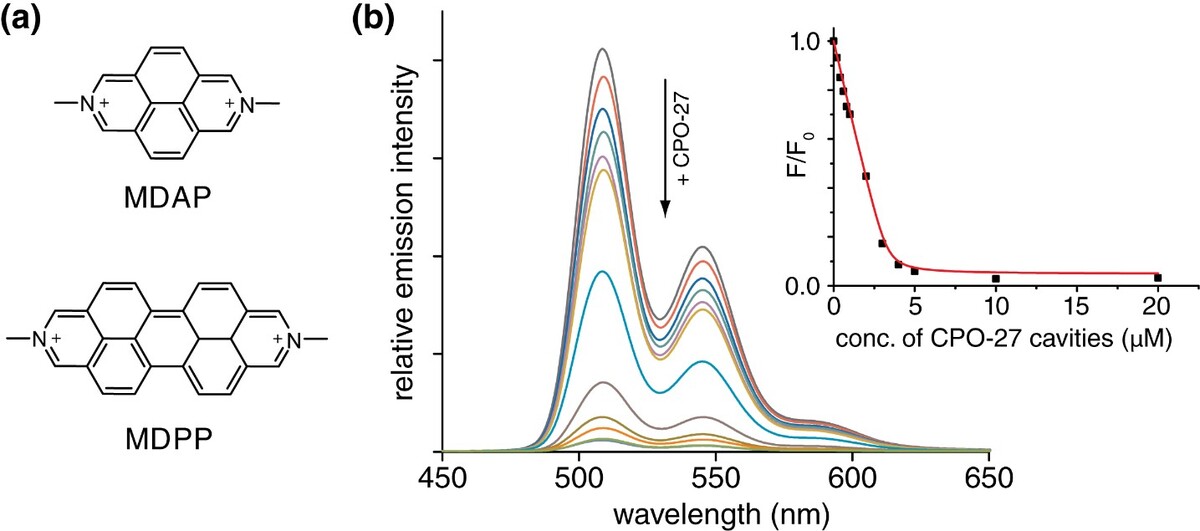
First, we synthesized two different MOF classes, namely CPO-27 and ZIF-8 (Fig. 1). The candidates were chosen for the stability in water, similar chemical structure, but differing pore sizes. We then screened for suitable analytes to be encapsulated by the MOF structures. To follow the analyte uptake by sensitive spectroscopic methods, a range of fluorescence dye molecules was first tested. Out of many, the dyes MDAP and MDPP (Fig. 2) showed the highest fluo rescence response upon encapsulation by CPO-27.
Through titration, we extracted the thermo dynamic binding constants for both dyes. The values are high compared to their affinities with non-charged hosts in water. Hence, the extra binding stabilization by the non-classical hydro phobic effect seems to play a role. Eventually, the dyes bound so tightly, as not be replaced by com petitive binders like salts, amino acids, or steroids. However, first evidence for co-binding of small analytes was observed. Consistent with the sus pected dye-uptake (as opposed to a dye-surface binding), the dyes did not show spectroscopic changes in the presence of the smaller pore-sized ZIF-8. As a result of the high binding affinity, we also observed remarkably slow uptake kinetics (~30 min). Though, hampering direct calorimet ric investigations, they can be of great practical utility for the design of non-equilibrium based chemical sensors (patent submitted by KIT). In short, nanoporous MOFs proved to be excellent platforms to study analyte-receptor interactions in aqueous media and offer great potential for developing wet-sensing devices.