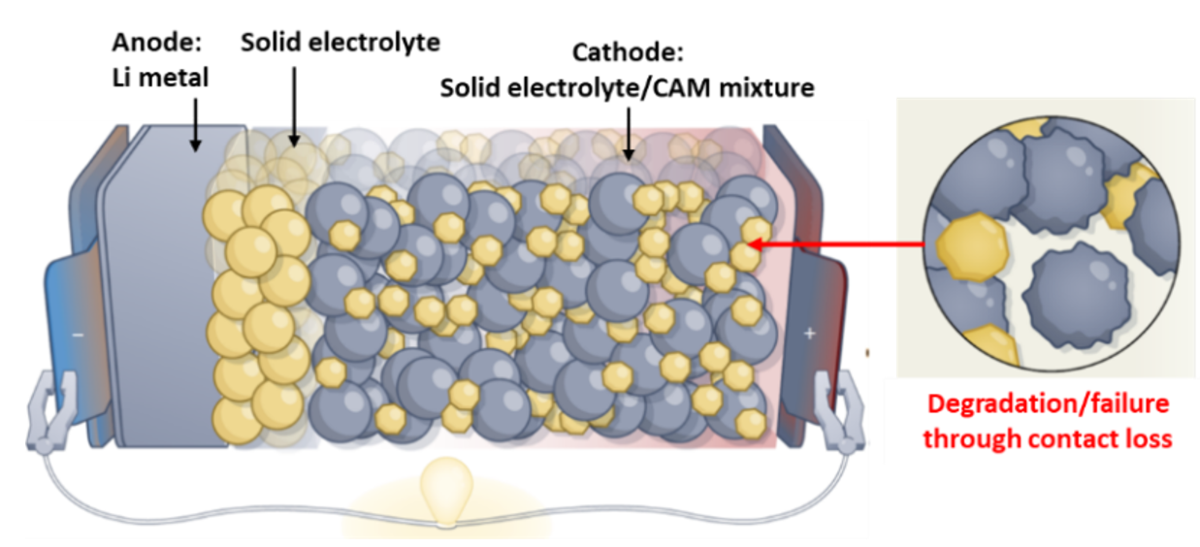
Can the mitigation of cathode volume changes enable stable solid-state batteries?
There is a rising global demand for rechargeable energy storage technologies such as the Li-ion battery, particularly owing to the increasing number of electric vehicles and portable electronics demands. However, consumers still request devices with higher energy- and power densities related to fast charging times and long-range vehicles. Solid-state batteries a are a potential enabler for such demands, however, several hurdles pertaining to their lifetime and scalable fabrication among others still need to be overcome.
State-of-the-art Li-ion batteries utilize organic liquid electrolytes which pose two major issues. First, they offer a limited temperature window for battery operation due to the volatility of the flammable liquid electrolyte, also raising safety concerns. Second, they are incompatible with metallic Li anodes preventing their implementation which could offer a strongly increased gravimetric capacity. Replacing the liquid- with an inorganic solid electrolyte (SE) could overcome both issues, and therefore, the development of solid-state batteries (SSB, Fig. 1) is currently pursued in academic and industrial research.1,2 Sulfide-based SEs are especially attractive because of their high Li-conductivity, comparable or even exceeding that of liquid electrolytes, and their mechanical softness allowing intimate contact with electrode materials.2 However, the application of a SE causes other detrimental (electro)chemical and (chemo)mechanical processes which negatively affect battery performance and lifetime. Cathode (positive electrode) active materials, which form a composite mixture with the SE (Fig. 1), usually undergo significant volume changes during Li (de)intercalation (i.e., battery charge/discharge). Unlike liquid electrolytes, the rigid nature of the SE leads to a (chemo)mechanically driven separation and contact loss between the cathode active material and the SE and thus causes severe battery performance decay.2,3 There is currently a lack of mechanistic understanding in the precise correlation between volumetric changes in cathode active materials and resulting phase separation with solid electrolytes because of the simultaneous occurrence of several degradation effects in solid-state batteries, rendering proper experiment design essential.
With the financial support of the YIN award Dr. Florian Strauss and Dr. Simon Fleischmann want to answer the question: How does (chemo)mechanical degradation in solid-state batteries affect battery performance?
Florian as an inorganic chemist is working in the field of solid-state batteries and solid electrolytes and Simon is a materials scientist specialized in electrode materials synthesis and characterization. They teamed up to bring together both their expertise in solid-state chemistry, electrochemistry and advanced materials characterization. Simon and his team specifically design layered cathode materials allowing to control their (chemo)mechanical behavior during Li (de)intercalation, which are integrated into solid-state batteries with suitable solid electrolytes with the help of Florian’s team. The degraded SSB cells will be investigated “post mortem” with the help of cryogenic electron microscopy techniques to analyze degradation as a function of (chemo)mechanical behavior. Currently, electrochemical studies are underway investigating different cathode/SE model systems which allow for a separation between (electro)chemical and (chemo)mechanical effects in solid-state batteries.
References
(1) Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces. Chemical Reviews 2020, 120 (14), 6820–6877. https://doi.org/10.1021/acs.chemrev.9b00268.
(2) Janek, J.; Zeier, W. G. Challenges in Speeding up Solid-State Battery Development. Nature Energy 2023, 10.1038/s41560-023-01208–01209. https://doi.org/10.1038/s41560-023-01208-9.
(3) Banerjee, A.; Wang, X.; Fang, C.; Wu, E. A.; Meng, Y. S. Interfaces and Interphases in All-Solid-State Batteries with Inorganic Solid Electrolytes. Chemical Reviews 2020, 120 (14), 6878–6933. https://doi.org/10.1021/acs.chemrev.0c00101.